Protein Folding
|
The
native conformation of a protein in stable in a narrow range of temperature,
pH, and other solvent conditions. A change in temperature, pH, or the
addition of denaturants (such as urea, guanidinium hydrochloride),
denatures/unfolds the protein. The changes of the free energy, enthalpy and
entropy during the folding/unfolding reactions are studied to understand the
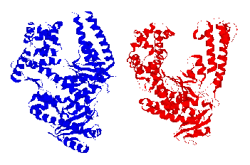
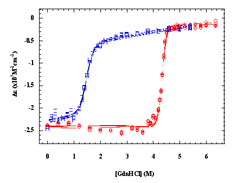
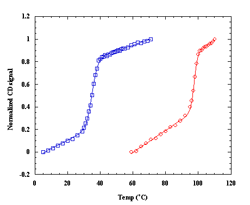
energetics over the proteins. Our lab has examined the thermal and chemical denaturation of the Klentaq and Klenow, large fragment domains of the Type I polymerases from Thermus aquaticus (Taq) and Escherichia coli (Pol I). Taq polymerase is both structurally and functionally homologous to E. Coli Pol I DNA polymerase, yet Taq is known to retain at least partial activity at temperatures up to 97.5°C, which is the basis of its use in the polymerase chain reaction (PCR). Taq and E. Coli Pol I DNA polymerases are both single polypeptide chains comprised of three structure/function domains: a polymerization domain, a proofreading domain (inactive in Taq), and 5' nuclease domain. Removal of the 5' nuclease domain yields the Klentaq and Klenow “large fragments” of each polymerase, both of which are active polymerases on their own. Klentaq and Klenow polymerases have highly homologous tertiary structures (Figure 1). Klentaq has one of the largest free energies (27 kcal/mol) of unfolding among monomeric proteins yet characterized, and that the stabilization free-energy difference (~20 kcal/mol) between Klentaq and Klenow is one of the largest yet characterized for a homologous thermophilic-mesophilic protein pair. Figure 2 is the guanidine hydrochloride denaturations of Klenow and Klentaq. Individual data points and fits to the linear extrapolation model are shown for the CD signals at 218, 219, 220, 221 nm at 25°C. Klentaq is fully reversible upon the chemical denaturation across the temperatures range 5 to 75°C but Klenow can only be partially recovered after denaturation. Thermal denaturation yields DH and Tm values of ~240 kcal/mole and 99°C for Klentaq, and ~120 kcal/mole and 36°C for Klenow. Figure 3 is the thermal denaturation of Klenow, and Klentaq monitored by CD spectroscopy. Each point presents one data scanned and lines are fits to a modified Van’t Hoff equation. To further understand the thermodynamic stability differences between these two homologous polymerases, the protein stability curves are being constructed. Solution structural characterization of the denatured Klentaq and Klenow are also studied with the uses of analytical ultracentrifugation (AU), dynamic light scattering (DLS), and small angle X-ray scattering (SAXS).
|
|
|
|
|
|
|
|
References: Schoeffler AJ, Joubert AM, Peng F, Khan F, Liu C and LiCata VJ. Extreme free energy of stabilization of Taq DNA polymerase, Proteins: Structure, Function, and Bioinformatics 2004; 54, 616-621 Richard, A.J., Liu, C.C., Klinger, A.L., Todd, M.J., Mezzasalma, T.M., and LiCata, V.J. Thermal stability landscape for Klenow DNA polymerase as a function of pH and salt concentration, Biochem. et. Biophys. Acta. 2006 1764: 1546-1552 Liu, C.-C., Yang, Y., and LiCata, V.J., Origins of the thermostability of Taq DNA polymerase: entropy and denatured state size, submitted. |